Acoustic Output Testing
Acoustic output testing, measurement and reporting follows the IEC 62359 standard (Ultrasonics - Field Characterization - Test Methods For The Determination Of Thermal And Mechanical Indices Related To Medical Diagnostic Ultrasonic Fields), the IEC 60601-2-37 standard (IEC 60601-1 Amendment 1 - Medical Electrical Equipment - Part 2-37: Particular Requirements For The Basic Safety And Essential Performance Of Ultrasonic Medical Diagnostic And Monitoring Equipment) and the FDA guidance: Marketing Clearance of Diagnostic Ultrasound Systems and Transducers, June 27, 2019.
Sigma Biomedical (Sigma Scientific Services LLC) is an A2LA accredited laboratory under the ISO/IEC 17025 standard for acoustic test methods. Click Here for our accreditation certificate. Sigma Scientific Services is an FDA accredited safety and performance medical device testing laboratory under the ASCA program.Our offer
We offer a complete acoustic output measurement service. We will carry out acoustic output measurement in autoscanning and/or non-autoscanning modes and provide the test report to use in the 510(k) submission to the FDA Office of Device Evaluation, Center for Devices and Radiological Health, or to the European Medical Device Directive (MDD). Additional measurements include acoustic property measurements of ultrasonic gels such as acoustic attenuation. Typically there is a minimum of 3 transducers per imaging mode that need to be tested for statistical significance. However, the number of transducers might increase depending on how close the reported value is to FDA pre amendment values as increasing the number of measurements reduces the standard deviation and thus the maximum reported value.
Test Report
Test Reports include maximum acoustic field variables, statistical and uncertainty analysis, a description of the measurement system, description of the measurement process, and hydrophone calibration methods. Variables to be reported include Pulse Waveform and Pulse Intensity Integral (PII) Plots, Zsp - Position of the maximum spatial PII. Center Frequency, Spatial Peak Pulse Average Intensity (Isppa), Spatial Peak Temporal Peak Intensity, Spatial Peak Temporal Average Intensity (Ispta), Intensity Maximum (Imax) values, Pulse Duration, In-Water Measured Intensity Values Derated (estimated in situ) Intensity values, Pressure Positive Peak and Pressure Negative Peak values, Acoustic Power, Beam Dimensions. We have provided test reports to agencies in the US (FDA), Canada, Japan, and Europe for diagnostic ultrasound product registration.
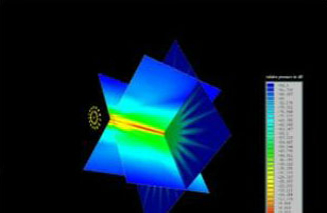
3D Beam Simulation
Ultrasonic Fields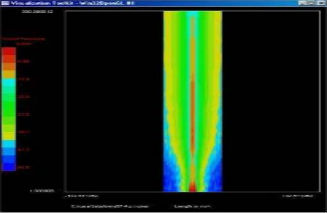
2D Beam Measurement
Ultrasonic Fields
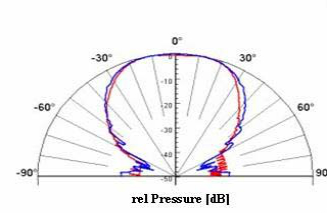
Beam Profile
Acoustic Report